Fabiana Fatiwaki
Atualizado março - 2022
Epidemiologia
As Infecções Relacionadas à Assistência à Saúde (IRAS) consistem em eventos adversos infecciosos que são adquiridas durante a prestação dos cuidados de saúde. Portanto, pode-se adquirir uma infecção, no hospital, nos serviços de hemodiálise, na assistência domiciliar, em procedimentos ambulatoriais e nas clínicas odontológicas. As principais IRAS são: Infecção do Sítio Cirúrgico, Pneumonia, Infecção do trato Urinário e Infecção da Corrente Sanguínea.
As infecções do sítio cirúrgico (ISC) são uma infecção comum associada à assistência à saúde (IRAS). Segundo o Center for Disease Control and Prevention, as infecções do sítio cirúrgico são definidas como infecções que ocorrem 30 dias após a cirurgia sem implante, ou dentro de 90 dias se um implante for colocado e a infecção estiver relacionada à cirurgia.
Magnitude do problema
Embora tenham sido feitos avanços nas práticas de controle de infecção, incluindo melhorias na ventilação da sala operatória, métodos de esterilização, barreiras, técnica cirúrgica e disponibilidade de profilaxia antimicrobiana, as Infecções de Sítio Cirúrgico (ISC) continuam sendo uma causa substancial de morbidade, hospitalização e óbito, além do que nos EUA:
É responsável por 20% de todas as IRAS e está associada a um aumento de 2 a 11 vezes no risco de mortalidade.
É o tipo de IRAS mais caro, com um custo anual estimado de US$ 3,3 bilhões.
Prolonga o tempo de permanência hospitalar em 9,7 dias.
Custo da hospitalização aumentado em mais de US$ 20.000 por admissão.
No Brasil, a ISC ocupa a terceira posição dentre as infecções encontradas nos serviços de saúde e compreende de 14 a 16% das infecções dos pacientes hospitalizados. Foi realizado Inquérito Nacional de Prevalência de Infecções Relacionadas à Assistência à Saúde (IRAS) (2011-2013) com a participação de 152 hospitais das 5 (cinco) macrorregiões. Os hospitais foram classificados segundo o tipo de porte, em grande (≥ 250 leitos), médio (50 -199 leitos) e pequeno porte (< 50 leitos). Um total de 6520 pacientes participou do estudo.
O resultado encontrado foi: o total de IRAS foi de 10,8 %. Infecções do sítio cirúrgico foram encontradas em 1,5% da amostra total, entretanto, quando analisados os pacientes que foram submetidos a procedimentos cirúrgicos, a ISC foi 9,8%. Vale salientar que esses dados são sem vigilância pós-alta hospitalar.
As ISC estão associadas às internações hospitalares mais prolongadas, a procedimentos cirúrgicos adicionais, a tratamento em unidades de terapia intensiva, e maior mortalidade. As ISCs geram impacto muito forte na economia da saúde de todos os países, sobretudo em países em desenvolvimento.
Estima-se que 40% a 60% de ISCs pode ser evitada com o uso de medidas baseadas em evidências. Um extensivo programa de vigilância pode reduzir as taxas de infecções de sítio cirúrgico, mas para que este programa seja efetivo deve-se conhecer a real incidência destas infecções e os fatores de risco associados. Nos EUA, as ISCs tornaram-se uma métrica de pagamento por desempenho, que vinculam remuneração com a melhoria da qualidade no serviço prestado.
Para controlar as ISC e estabelecer medidas de prevenção, é necessário identificar os fatores de risco que contribuem para o desenvolvimento da infecção. Conhecer esses fatores é importante para o planejamento e implementação de ações que permitam reduzir a incidência das ISC. Toda cirurgia traz um risco, devido a uma interação complexa entre fatores relacionados ao paciente, relacionados como procedimento e relacionados com a microbiota. Por isso é importante levar em conta que cada paciente oferece uma multiplicidade de fatores que podem alterar seus mecanismos de defesa sistêmica.
Fatores de risco do paciente, que podem influenciar o de desenvolvimento de ISC
Idade – a pele não está excluída do complexo processo de envelhecimento. O suprimento nervoso e vascular da pele diminui com a idade da pessoa, essas alterações fisiológicas predispõem à lentidão ou má cicatrização de feridas em idosos.
Tempo de internação pré-operatória – preferencialmente, o paciente deverá internar no mesmo dia do procedimento cirúrgico ou no dia anterior.
Paciente – colonizado por microrganismos resistentes.
Cirurgia de emergência /urgência – tem maior risco de eventos adversos, incluindo ISC.
Diabetes – acarreta no paciente alterações fisiopatológicas da cicatrização, complicações vasculares, neuropáticas e efeitos inibitórios do mecanismo de defesa.
Obesidade – considerada fator de risco significativo devido à baixa perfusão de oxigênio e ao aumento da duração do procedimento cirúrgico em função do excesso de tecido adiposo , que dificulta o acesso a estruturas a serem operadas.
Terapia para câncer – quimioterapia e radioterapia aumentam o risco subsequente de ISC. Retardar o procedimento eletivo reduz o risco de ISC, entretanto essa medida nem sempre é factível.
Terapia Imunosupressiva – ela retarda a cicatrização da ferida, porém não está diretamente relacionada ao desenvolvimento de ISC. Por exemplo, corticoterapia.
Coexistência de infecção em sítio remoto – tratar toda infecção ativa remota, antes da do procedimento cirúrgico, sobretudo em procedimentos em que será implantado material protético.
Desnutrição – hipoalbunemia aumenta risco de ISC comparada a taxa de albumina normal.
Tabagismo – o tabagismo está associado a efeitos adversos significativos após a cirurgia, incluindo infecção do sítio cirúrgico e complicações pulmonares. O efeito deletério do tabagismo na cicatrização de feridas é multifatorial, com mecanismos que incluem vasoconstrição levando à isquemia relativa dos tecidos operados, diminuição da resposta inflamatória e alterações no metabolismo do colágeno. Parar de fumar 4 a 6 semanas é recomendado antes da cirurgia para reduzir o risco de ISC e de complicações pulmonares.
Dessa forma, deve-se realizar avaliação completa de todos os pacientes cirúrgicos no pré-operatório e atuar nos fatores que podem ser modificáveis, tais como; reduzir a hospitalização pré-operatória; avaliação e tratamento de infecções remotas; interrupção do uso de tabaco (abstenção pelo menos 30 dias antes da realização da cirurgia), controle do nível de glicemia pacientes diabéticos e não diabético, entre outros. Em relação as medidas relativas ao perioperatório, intraoperatório e pós-operatório estão descritas no texto.
Definição de Procedimento cirúrgico
Ocorre quando existe pelo menos uma incisão (incluindo abordagem laparoscópica e orifícios de broca craniana), realizado em centro cirúrgico(sala de cirurgia, sala de cesariana ou sala de radiologia intervencionista), feita através da pele, membrana mucosa, ou de uma incisão deixada aberta durante um procedimento cirúrgico anterior.
Etiologia da Infecção do Sítio Cirúrgico
A fonte mais comum de agentes patogênicos para a maioria das ISC é a flora endógena da pele do paciente, membranas mucosas ou vísceras ocas, pois ao se incisar a pele ou membranas mucosas, esses tecidos são expostos, podendo ocorrer o risco de contaminação.
A contaminação por fontes exógenas de ISC – pode ocorrer por transmissão de microrganismos pela equipe cirúrgica, por instrumentos cirúrgicos ou o ambiente durante o ato cirúrgico.
Classificação da Cirurgia segundo potencial de contaminação
A classificação de acordo com o potencial de contaminação da ferida é utilizada para estratificar o risco de ISC e serve de base como indicador de qualidade da assistência ao paciente.
As taxas segundo o potencial de contaminação de ISC são:
Cirurgia Limpa: risco de ISC < 2%.
Limpa – contaminada: risco de ISC (< 10%).
Contaminada: risco de ISC (em torno de 20%).
Cirurgias Limpas
São cirurgias realizadas em tecidos, na ausência de infecção e nenhum sinal de inflamação local e em que não ocorreram penetrações no Trato Respiratório, Trato Gastrointestinal, Genital e Urinário (vísceras ocas). São primariamente fechadas e, se necessário, drenar, utilizarreno fechado. Cirurgias que acompanham o traumatismo não penetrante (fechado) devem ser incluídas nessa categoria se obedecerem aos critérios acima.
Exemplos: Hérnias, cirurgias da tireóide, cirurgias cardíacas, neurocirurgia, artroplastia do quadril, cirurgias ortopédicas eletivas, mastectomia parcial e radical, cirurgia de ovário, esplenectomia, cirurgia vascular, etc. Esplenectomia realizada após trauma fechado – é considerada limpa.
Cirurgias Limpas Contaminadas
São cirurgias em que ocorre penetração no Trato Respiratório, Trato Gastrointestinal, Genital e Urinário sob condições controladas e rara contaminação no perioperatório (sem contaminação significativa) e sem infecção.
Exemplos: histerectomia abdominal, intestino delgado, cirurgias de vias biliares sem estase ou obstrução biliar, cirurgia gástrica e duodenal, apêndice, vagina e a orofaringe.
Cirurgias contaminadas
São feridas abertas, recentes e acidental. Em adição, operações com grande quebra da técnica asséptica (por exemplo, massagem cardíaca externa) ou derramamento grosseiro de conteúdo gastrointestinal na cavidade abdominal e em tecidos onde há processo inflamatório agudo, sem secreção purulenta, incluindo necrose tecidual (gangrena seca) são incluídas como cirurgias contaminadas. Exemplos: cirurgia do cólon, intranasal, bucal e dental.
Cirurgias infectadas
Feridas traumáticas antigas com presença de tecido desvitalizado, corpo estranho, contaminação fecal ou infecção clínica presente (purulenta) ou vísceras ocas perfuradas.
Definição de Infecção do Sítio Cirúrgico (ISC)
São definidas como infecções que ocorrem 30 dias após a cirurgia sem implante, ou dentro de 90 dias se um implante for colocado e a infecção estiver relacionada à cirurgia.
Data da infecção para ISC: a data da infecção é a data do procedimento cirúrgico.
Classificação e critérios definidores de infecção de Sítio Cirúrgico – ISC
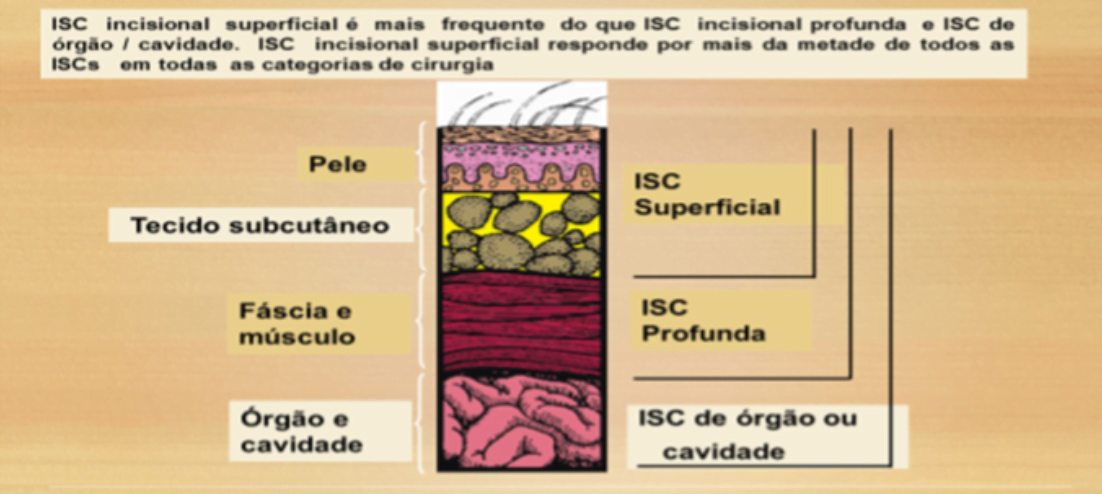
ISC - Incisional Superficial
Ocorre nos primeiros 30 dias após o procedimento cirúrgico (sendo o primeiro dia a data do procedimento) e envolve apenas pele e tecido subcutâneo e apresenta pelo menos um (1) dos seguintes critérios:
Drenagem purulenta da incisão superficial;
Cultura positiva de secreção ou tecido da incisão superficial, obtido assepticamente2;
A incisão superficial é deliberamente aberta pelo cirurgião na vigência de pelo menos um dos seguintes sinais e sintomas: dor, aumento da sensibilidade, edema local, hiperemia ou calor.
Diagnostico de infecção incisional superficial pelo cirurgião ou outro médico assistente.
Observações:
Não definidos como ISC superficial (o abscesso: inflamação mínima ou drenagem confinada aos pontos da sutura).
Não serão considerados os resultados de culturas positivas, quando coletadas através de SWAB (hastes com ponta de algodão).
ISC Incisional Profunda
Ocorre nos primeiros 30 dias após a cirurgia(sendo o primeiro dia a data do procedimento) ou até 90 dias, se houver colocação de implantes, envolve tecidos moles profundos à incisão ex: (facial e ou músculos) e apresenta pelo menos um (1) dos seguintes critérios:
Drenagem purulenta da incisão profunda, mas não originada de órgão/cavidade;
Deiscência espontânea profunda ou incisão aberta pelo cirurgião e cultura positiva ou não realizada, quando o paciente apresentar pelo menos 1(um) dos seguintes sinais e sintomas: febre (temperatura ≥38°C), dor ou tumefação localizada;
Abscesso ou outra evidência de infecção envolvendo tecidos profundos, detecta do durante exame clinico, anatomopatológico ou de imagem;
Diagnostico de infecção profunda/superficial feito pelo cirurgião ou outro médico assistente.
ISC Órgão Cavidade
Ocorre nos primeiros 30 dias após a cirurgia ou até 90 dias, se houver colocação de implantes, envolvem qualquer órgão ou cavidade que tenha sido aberta ou manipula durante a cirurgia e apresenta pelo menos Um (1) dos seguintes:
Cultura positiva de secreção ou tecido do órgão/cavidade obtido assepticamente;
Presença de abscesso ou outra evidencia que a infecção envolve os planos profundos da ferida identificada em reoperação, exame clínico, anatomopatológico ou de imagem;
Diagnostico de infecção de órgão/cavidade pelo médico assistente.
Observação:
Toda infecção do trato urinário após cirurgia urológica será considerada ISC – Órgão/Cavidade.
Os critérios definidores de Um (1) sítio específico de ISC-Órgão/Cavidade estão no capítulo 1 do Manual de Critério e Diagnósticos publicados pela Anvisa.
Observações:
Qualquer ISC (incisional superficial, incisional profunda ou órgão/cavidade) relacionadas a: cirurgia cesariana, implante de prótese mamária, implante de prótese de quadril primária, implante de prótese de joelho primária, derivações internas neurológicas e pós-revascularização do miocárdio deve ser notificada. No entanto, quando identificado mais de um tipo de ISC relacionada a um desses procedimentos cirúrgicos computar e notificar o tipo mais grave. Exemplo: após uma cirurgia cesariana, foi identificada (fechado o critério diagnóstico) uma ISC incisional profunda e uma infecção uterina (ISC/OC). Nesse caso será computado apenas a infecção uterina (ISC/OC).
Exemplo de Infecção de Sítio Cirúrgico Incisional Superficial

Interpretação:
Os elementos que definem o critério ocorrem dentro dos 30 dias após a cirurgia e são suficientes para definir uma ISC superficial.
A data da infecção: 04/03, que é a data do procedimento cirúrgico.
Recomendações Relacionadas ao Centro Cirúrgico
É constituído de um conjunto de áreas e instalações que permitem efetuar as intervenções cirúrgicas nas melhores condições de segurança para o paciente, e de conforto para a equipe integrada que o assiste. Estruturalmente, no centro cirúrgico, dentre outros fatores, o sistema de ventilação tem dois objetivos: climatização (conforto térmico para o paciente e equipe cirúrgica) e redução da contaminação sítio operatório e dos equipamentos cirúrgicos.
São estas as condições recomendadas para salas cirúrgicas
Fluxo de ar na sala cirúrgica deve ser introduzido próximo ao teto e com exaustão do ar próximo ao piso, com pelos menos 15 trocas de ar por hora, sendo 3 de ar fresco.
Os filtros e dutos do sistema devem passar por serviços periódicos de manutenção preventiva, justamente para garantir dia após dia o máximo de qualidade na limpeza e refrigeração do ar.
A variação de temperatura deve ser de 20 a 24°C.
A umidade do ar deve ficar entre 50% no mínimo e no máximo com 60%.
Ter termômetro de máxima e mínima.
Deve-se manter a porta da sala fechada durante o ato cirúrgico cuja finalidade é assegurar a pressão positiva na sala de cirurgia em relação às áreas adjacentes.
Reduzir o número de circulação de pessoas e evitar abrir a porta constantemente, a não ser que seja estritamente necessária.
Preparar a mesa com material cirúrgico o mais próximo do início da cirurgia e inspecionar materiais esterilizados no que tange ao controle de qualidade.
Uma equipe multidisciplinar, com um coordenador responsável, deve ser estabelecida no Centro Cirúrgico, para alinhar as diretrizes de limpeza e desinfecção antes do primeiro procedimento do dia, entre os procedimentos e após a realização do ultimo procedimento do dia, nas áreas perioperatórias, dos equipamentos críticos e não críticos utilizados na sala de cirurgia. Uma abordagem programática, com foco em: treinamento dos servidores em relação aos diversos processos, auditoria com feedback dos resultados encontrados, para melhor adesão da equipe multidisciplinar.
Não levar para dentro da sala de cirurgia bolsas e equipamentos eletrônicos.
Lavar as mãos com água e sabão neutro – toda a equipe deve lavar as mãos antes de entrar na área privativa do Centro Cirúrgico.
Remover todos os adornos das mãos e antebraços como: anéis, relógios e pulseiras, antes de iniciar a antissepsia das mãos. Estudos mostram maior potencial para contaminação quando os adornos são mantidos, por impedirem a correta higienização das mãos;.
É proibido o uso de unhas artificiais;
O uso correto da máscara (cobrindo boca e nariz) e do gorro (cobrindo completamente os cabelos) é obrigatório para todos os membros da equipe anestésica e cirúrgica, ao entrar na sala cirúrgica e deve permanecer dessa forma durante todo o procedimento cirúrgico.
Não há evidências de que os propés previnam a contaminação do ambiente e da ferida cirúrgica. Logo, não se recomenda o uso de propés no ambiente cirúrgico. Recomenda-se o uso de sapato limpo e fechado para proteção individual do profissional de saúde, no que tange ao risco ocupacional.
Diante de cirurgias onde houver grande exposição aos componentes químicos da fumaça cirúrgica produzida pelo eletrocautério pode ser utilizada a máscara N95, além disso, a instalação de exaustores de ambiente e aspiradores próprios para a fumaça cirúrgica, minimiza os riscos referentes à inalação dessa fumaça preservando a saúde ocupacional da equipe cirúrgica.
Intervenções e Recomendações nas diversas fases do Ato Cirúrgico, baseadas em Evidências Científicas:
No ano de 2016, o Colégio Americano de Cirurgiões, (atualizou o guia de ISC baseado opinião de especialista). Em 2016, a Organização Mundial da Saúde (OMS), em 2017, o Center Disease of Controland Prevention – CDC, em 2018, a Associação Espanhola de Cirurgiões (ACE) e em 2019, o for Health and Care Excellence – NICE, realizaram revisão sistemática da literatura com o intuito de atualizar as recomendações descritas para prevenção e controle das ISC. Algumas medidas foram acrescentadas ou ratificadas com a literatura cientifica publicada recentemente.
Recomendação 1 - Banho Pré-operatório
Sabão neutro deve ser realizado na noite anterior e na manhã da cirurgia, para todas as cirurgias. Para cirurgias ortopédicas, neurológicas e cardíacas – quando se indicar o banho com clorexidina degermante deve ser seguido o protocolo descrito no texto*.
Banho com de Gluconato de Clorexidina*
É importante seguir a técnica de aplicação do banho com de Gluconato de Clorexidina com base degermante a 4%, descrita abaixo:
Perguntar ao paciente se possui alergia a esse produto. Em caso de pacientes alérgicos, não realizar o banho;
Realizar banho com água morna;
Lavar os cabelos com água morna;
Caso o paciente, use sabão ou xampu regular, deve-se certificar que o sabão ou xampu usados foram removidos completamente, pois o Gluconato Clorexidina degermante a 4% é inativado por sabonetes e xampus.
Não aplicar em face, olhos, boca, nariz e ouvidos;
Não utilizar esponja para aplicação da Clorexidina;
Após tomar o banho, desligar o chuveiro e aplicar com as mãos a solução de 100 ml de Gluconato de Clorexidina degermante a 4%, do pescoço para baixo, sobretudo na região a ser operada, nas axilas, pregas mamárias, regiões inguinais, órgãos genitais e região anal;
Deixar secar a espuma por 2 minutos antes de enxaguar;
Não utilizar desodorante, creme e algum tipo de pó ou loção, pois inativa a Clorexidina;
Utilizar roupas e toalhas limpas após o banho com Clorexidina.
ATENÇÃO:
Em caso de pacientes com mobilidade reduzida, pode ser usado o lenço umedecido composto por Gluconato de Clorexidina degermante a 2%.
A Clorexidina apresenta um importante efeito cumulativo de modo que sua ação antimicrobiana aumenta com seu uso periódico.
Ao aplicar o produto sobre a pele e ocorrer alguma reação alérgica, deve-se suspender imediatamente e procurar orientação médica.
Recomendação 2 - Pesquisa nasal para S. aureus e descolonização de portadores
Não há consenso na literatura para se fazer pesquisa nasal e descolonização de rotina para pacientes submetidos a cirurgia. O risco de infecção em portadores nasal de S. aureus, tem sido estudado principalmente em pacientes cirúrgicos das seguintes especialidades, ortopedia, cardíaca e neurocirurgia com implantes e pacientes imunossuprimidos. Colonização prévia é fator de risco pré-operatório para infecção de sítio cirúrgico.
Não há um regime padrão para descolonização de pacientes com S.aureus nasal. Pacientes com colonização nasal sabida por S. aureus que serão submetidos as cirurgias(ortopedia, cardíaca e neurocirurgia com implantes e pacientes imunossuprimidos), aplicar na face anterior de cada narina)a pomada de mupirocina a 2% duas vezes ao dia por cinco dias combinadas ou não à lavagem corporal com gluconato de clorexidina degermante (4%) diariamente por cinco dias. Sugere considerar este tratamento também a pacientes que sabidamente são portadores nasais de S. aureus e que sejam submetidos a outros tipos de cirurgia.
Recomendação 3 - Preparação mecânica do cólon e uso de antibióticos orais
Recomenda-se que a preparação mecânica do cólon isolada (sem administração de antibióticos orais) não seja utilizada para a redução de ISC sem pacientes adultos submetidos a cirurgia colorretal eletiva.
Recomendação 4 - Tricotomia
Pacientes que irão se submeter a qualquer procedimento cirúrgico, cabelos/pelos não devem ser removidos ou, se estritamente necessária, realizar com tricomizador elétrico com lâmina descartável. Não realizar na sala de cirurgia.
Recomendação 5 - Antissepsia das mãos da equipe cirúrgica
Recomendações na utilização de antissépticos:
Verificar as datas de validade, indicar a data de abertura do frasco e conservar segundo as recomendações ao abrigo da luz e do calor (principalmente se for inflamável) checar a duração de utilização após abertura do produto e se o frasco fica bem fechado.
Toda a equipe cirúrgica deverá higienizar as mãos com sabonete neutro e água ao chegar no centro cirúrgico, após ter vestido a roupa privativa (toca e máscara).
Deve ser realizada com antisséptico degermante à base de Polivinilpirrolidona-iodo a 10%; (PVP-I) ou Clorexidina a 2% (GHG)- toda a equipe cirúrgica.
Preparo da pele da equipe cirúrgica, tem como finalidades:
Redução do número de bactérias.
Remover todos os adornos das mãos e antebraços como: anéis, relógios e pulseiras, antes de iniciar a antissepsia das mãos;
É proibido o uso de unhas artificiais;
Manter unhas curtas;
Atualmente, quase todos os estudos desencorajam o uso de escovas, que não são mais recomendadas para preparação pré-operatória das mãos, uma vez que a utilização de esponja descartável reduziu a contagem bacteriana nas mãos tão eficazmente quanto esfregar com uma escova. A própria Organização Mundial da Saúde (OMS) não recomenda o uso de escovas para essa finalidade devido a seu efeito abrasivo.
Técnica
Abrir a torneira, molhar as mãos, antebraços e cotovelos;
Recolher, com as mãos em concha, o antisséptico Degermante de Clorexidina a 2% ou 4% espalhar nas mãos, antebraço e cotovelo. No caso de esponja impregnada com antisséptico, pressione a parte da esponja contra a pele e espalhe por todas as partes;
Limpar sob as unhas com esponja sob a água corrente;
Friccionar as mãos, observando espaços interdigitais e antebraço por no mínimo 2 minutos, mantendo as mãos acima dos cotovelos;
Enxaguar as mãos em água corrente, no sentido das mãos para cotovelos, retirando todo resíduo do produto. Fechar a torneira com o cotovelo, joelho ou pés, se a torneira não possuir foto sensor. Secar as mãos com compressas estéreis.
A qualidade da água do Centro Cirúrgico, deve ser assegurada para não ocorrer contaminação das mãos.
Recomendação 6 -Antissepsia das mãos da equipe cirúrgica com produto a base de álcool (PAB).
Segundo a OMS, o preparo cirúrgico das mãos com a solução degermante de Clorexidina ou PVP-I ou preparação alcoólica (fricção cirúrgicadas mãos com produto específico à base de álcool, sem enxague, também tem sido recomendado pela OMS, pois ambos os métodos são equivalentes na prevenção de ISC. Vale salientar que a eficácia do álcool depende de seu tipo, concentração e tempo de contato.
A preparação alcoólica tem ação rápida e efetiva contra os microrganimos, ação persistente e não há risco de recontaminação com a água da torneira. Com essa técnica se contribui para a economia de milhares de litros de água e do uso de compressa estéril. Além de contribuir para diminuir a resistência microbiana a clorexidina.
A fricção antisséptica das mãos com preparação alcoólica não realiza remoção de sujidades.
Técnica – Fricção antisséptica das mãos com preparação alcoólica.
Nas situações em que for necessário lavar as mãos com água e sabão líquido imediatamente antes da aplicação de PAB. As mãos devem ser secas com toalhas de papel não estéril, uma vez que a atividade das soluções alcoólicas é reduzida se as mãos não se encontrarem completamente secas;
Dispense em torno 3ml da solução na palma de uma mão. Com as pontas do dedo da mão oposta, friccione vigorosamente as pontas dos dedos, em seguida as mãos e antebraço até acima do cotovelo. Dispense em torno de 3 ml e repita na mão e antebraço do lado oposto.
Finalmente, dispense em torno de 3 ml da solução em uma das mãos e reaplique em ambas as mãos até o pulso.
Não enxugar (não usar compressas esterilizadas);
Manter os braços fletidos e voltados para cima e esperar secar completamente a solução alcoólica;
Calçar luvas estéreis na sala de cirurgia.
Respeitar as instruções do fabricante relativamente ao tempo de contacto recomendado, que deve corresponder ao tempo mínimo (2 minutos) que o produto deve estar em contacto com a pele na fase líquida até à sua completa evaporação.
Recomendação 7 - Degermação do sítio cirúrgico
Caso seja necessária se recomenda a seguinte sequência:
Umedecer a pele com solução fisiológica a 0,9% .
Friccionar a clorexidina degermante a 2% abrangendo área ampla e adjacente ao local da incisão.
Remover o resíduo com solução fisiológica a 0,9% estéril.
Após a degermação do sítio cirúrgico, realizar a antissepsia no campo operatório no sentido centrífugo circular (do centro para a periferia) e ampla o suficiente para abranger possíveis extensões da incisão, novas incisões ou locais de inserções de drenos, com solução alcoólica de PVPI -10% , com 1% de iodo livre ou solução alcoólica de clorexidina a 2% . Após, aplicar a solução alcoólica deixar secar naturalmente por evaporação.
Vale salientar que na literatura atual não há consenso entre se usar clorexidina ou PVP-I alcoólico na antissepsia do campo operatório, com a finalidade de prevenir Infecção do Sítio Cirúrgico. Entretanto, a maioria da literatura recomenda usar em procedimentos cirúrgicos a solução de clorexidina alcoólica a 2%, por meio de fricção e aguardar a secagem do produto para proceder à incisão.
Nota: 1 Não usar nenhum produto a base de álcool ou Clorexidina nos olhos ou ouvido, pois pode levar lesão de córnea e tímpano.
Nota: 2 Em relação ao globo ocular, fazer antissepsia periocular com solução aquosa de povidona a 10% com 1% de iodo livre (PVP-I tópico) .Após a antissepsia periocular, realizar antissepsia da córnea e da conjuntiva com colírio de iodopovidona a 5%, mantendo o antisséptico em contato com a área por no mínimo três minutos.
Nota: 3 Se a cirurgia for realizada em membranas mucosa, usar a solução antisséptica aquosa de Clorexidina a 2% ou Solução aquosa de PVP-I a 10% com 1% de Iodo livre.
Nota: 4 Ao se usar solução de Clorexidina aquosa ou alcoólica em cirurgia onde haverá contato com meninges ou cérebro deixar secar completamente, para evitar aracnoidite.
Observação: A clorexidina alcoólica a 0,5% é mais recomendada para ser usada em bloqueios neuromusculares, punção lombar, antissepsia para colher hemocultura, punção arterial ou venosa, nos rubs antes da troca dos equipos ou administração de medicamentos, entre outras.
Recomendação 8: – Antibioticoprofilaxia cirúrgica pré-operatória, quando indicada – o antibiótico deve ser administrado antes da incisão cirúrgica (dentro de 60 minutos), para todos os antibióticos, exceto para as quinolonas ou vancomicina que deve ser de 120 minutos antes da incisão cirúrgica. Vide protocolo publicado no HBDF para antibioticoprofilaxia cirúrgica pré-operatória para as diversas clínicas do hospital.
Recomendação 7 – Minimizar transfusões
A transfusão de sangue e hemoderivados no perioperatório está associada com a ocorrência de ISC em pacientes adultos submetidos a cirurgia eletiva.
Recomendação 8 - Emprego de uma técnica cirúrgica refinada.
É fundamental para prevenção de ISC (manutenção adequada da hemostasia, manipulação cuidadosa dos tecidos e órgãos, remoção de tecidos desvitalizados, evitar quebra de técnicas, uso criterioso de eletrocoagulação, entre outros).
Recomendação 9 -Controle glicêmico
Manter nível de glicemia < 200 mg /dL intraoperatório e perioperatório em diabéticos e não diabéticos.
Recomendação11 - Controle Normotermia (≥ 36ºC).
O uso de dispositivos de aquecimento do paciente deve ser iniciado no pré-operatório, mantido no intra e no pós-operatório.
Recomendação 12 - Oxigenioterapia
pacientes adultos submetidos a anestesia geral com intubação endotraqueal para procedimentos cirúrgicos se recomenda uma fração de 80% de oxigênio inspirado (FiO2) no intra-operatório e, se possível, no pós-operatório imediato por 2-6 horas.
Recomendação 13 - Campos plásticos
Não utilizar campos plásticos adesivos com ou sem propriedades antimicrobianas com o objetivo de prevenir ISCs.
Recomendação 14 - Dispositivos de proteção de feridas
Considerar o uso de dispositivos de proteção de feridas em cirurgias abdominais potencialmente contaminadas, contaminadas e infectadas.
Recomendação 15: Terapia profilática com pressão negativa
Se recomenda o uso de terapia profilática com pressão negativa em pacientes adultos em incisões cirúrgicas com fechamento primário, desde que sejam feridas de alto risco, com a finalidade de prevenção de ISC levando em consideração os recursos disponíveis.
Recomendação 16 - Utilização de luvas cirúrgicas
A Organização Mundial da Saúde (OMS), diz que muitos estudos nãodemonstram diferenças em relação a ISC associado ao uso de luvas duplas versus um único par de luvas, e não recomenda essa medida.
Entretanto, as luvas usadas na área de saúde desempenham um papel importante na proteção tanto dos pacientes como dos profissionais de saúde.O risco de exposição a patógenos transmitidos pelo sangue é uma das principais preocupações de cirurgiões e equipe cirúrgica.Segundo estudo publicado ao final do procedimento cirúrgico, em torno de 18% (com variação de 5% a 82%) das luvas cirúrgicas apresentam microperfurações, e, na maioria das vezes (80%), não é percebido pela equipe cirúrgica. Mesmo que na prática se use duas luvas, uma sobre a outra, perfurações ainda podem ser observadas em 4% dos casos após o procedimento cirúrgico.Os fatores contribuintes mais comuns para esse evento incluem perfurações com objetos pontiagudos, danos decorrentes de fragmentos ósseos e fricção com instrumentais complexos. Portanto, o uso de luvasduplas é recomendada para cirurgiasabertas, pelaAmerican Academy of Orthopaedic Surgery (AAOS), Centers for Disease Control and Prevention (CDC), The Occupational Safety and Health Administration American Academy of Orthopaedic Surgery, International College of Surgeons (ICS)e The National Institute for Health and Care Excellence (NICE).
Recomendação 17 – Suturas revestidas com antibióticos
Sugere o uso de suturas revestidas com triclosan independentemente do tipo de cirurgia.
Recomendação 18 - Tipos de Curativos
Curativos avançados versus curativos estéreis tradicionais – não utilizar nenhum tipo de curativo avançado em feridas cirúrgicas de fechamento primário e sim curativos estéreis tradicionais.
Recomendação 19 - Duração dos curativos
O curativo original deve ser deixado no local da cirugia por até dois dias (ou conforme orientação médica), ou seja, por 24 – 48 horas em ferida operatória com fechamento primário e mantida seca.Se o curativo ficar molhado de sangue ou qualquer outro líquido, deve ser trocado.
A vigilância das ISC é a pedra angular de qualquer programa de prevenção e controle de infecções.
A vigilância das ISC contribui para reduzir as taxas de incidência e monitorar a qualidade dos cuidados de saúde, tornando o atendimento ao paciente mais seguro. Coletar os dados sistematicamente fornecerá informações valiosas, necessárias ao longo do tempo, na aplicação de medidas preventivas e corretivas para reduzir os danos aos pacientes. Além disso, a coleta de dados padronizados permitirá que a instituição agregue, análise e relate o progresso para alcançar as metas estabelecidas na redução ISCs. Esses dados deverão ser conhecidos por toda a Instituição, na busca do envolvimento de todos para a melhoria da qualidade do paciente.
Indicadores para avaliação das medidas de prevenção e controle das ISC:
Taxa de Infecção em cirurgias limpas
Nº total de infecções em cirurgia limpa X 100
Número total de cirurgias limpas
Taxa de conformidade do antibiótico profilático administrado
Nº total do antibiótico profilático corretamente administrado dentro de 60 minutos, antes do início da cirurgia X 100
Nº total do antibiótico profilático administrado
Vigilância pós-alta das ISC
O acompanhamento pós-alta cirúrgico no monitoramento das ISC, deve ser realizado pelo NUCIH, a fim de melhorar a qualidade no atendimento, evitar também a subnotificação dos casos e aumentar a confiabilidade das taxas de ISC dos serviços. Ela pode ser realizada através de:
No retorno ao ambulatório;
Teleconsulta;
Busca fonada;
Por carta, e-mail ou outros meios.
Cirurgia Segura
Checklist de cirurgia segura é uma das ferramentas mais poderosas para evitar erros e eventos adversos.
A realização de um checklist de cirurgia segura é uma das ferramentas mais poderosas para evitar erros e eventos adversos. Um dos estudos pioneiros, publicado em 2009 no The New England Journal of Medicine, mostrou que a taxa de mortes e de complicações cirúrgicas caiu mais de 30% nos oito hospitais que participaram de um programa piloto da OMS para implantação de um checklist cirúrgico.
Referências Bibliográficas
CDC. NationalHealtcare Safety Network (NHSN) –Patient Safety Component Manual – January, 2022.
Mantoani CC, Margatho AS, Dantas RAS, Galvão CM, de Campos Pereira Silveira RC. Perioperative Blood Transfusion and Occurrence of Surgical Site Infection: Integrative Review. AORN J. 2019 Dec;110(6):626-634.
Link T. Guidelines in Practice: Preoperative Patient Skin Antisepsis. AORN J. 2022 Feb;115(2):156-166.
ANVISA. Critérios Diagnósticos de Infecção Relacionada à Assistência à Saúde. Agência Nacional de Vigilância Sanitária, Brasília-DF, Brasil, 2022.Acesso em: 15 de fevereiro de 2022.
ANVISA.Série Segurança do Paciente e Qualidade em Serviços de Saúde. Critérios Diagnósticos de Infecções Relacionadas à Assistência à Saúde,2.Capítulo 1, página 13.Acesso em: 15 de fevereiro de 2022.
ANVISA. Critérios Diagnósticos de Infecção Relacionada à Assistência à Saúde. Agência Nacional de Vigilância Sanitária, Brasília-DF, Brasil, 2017. Acesso em: 15 de dezembro de 2021.
ANVISA. Medidas de Prevenção de Infecção Relacionada à Assistência à Saúde. Agência Nacional de Vigilância Sanitária, Brasília-DF, Brasil, 2017.
ASSOCIATION OF PERIOPERATIVE REGISTERED NURSES. AORN guideline for surgical attire. In: Association of perioperative Registered Nurses. AORN Guidelines for Perioperative Practice. Denver, CO: AORN, Inc.; 2017. p. 105-128.
BADIA J M et al. A survey to identify the breach between evidence and practice in the prevention of surgical infection: Time to take action. International Journal of Surgery, Volume 54, Part A, June 2018, Pages 290-297.
BAN KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg. 2017; 224(1): 59-74.
CDC.Surgical Site Infection Event (SSI).NHSN Janeiro 2017. <https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf>. Acesso em: 15 de dezembro de 2021.
CDC. 608National Healthcare Safety Network (NHSN). Patient Safety Component Manual Chapter 9- Surgical site infection (SSI) event. 2018. p.9.Disponível em: https://pubmed. ncbi.nlm.nih.gov/27915053/. Acesso em: 15 de dezembro de 2021.
National Healthcare Safety Network (NHSN) Patient Safety Component Manual. Disponível em:<
https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pd>. Acesso em: 15 de dezembro de 2021.
DSPSV, M-C Roy. Michael Stevens. Guide to Infection Control in the Hospital Chapter 22: The Operating Room. International Society for Infectious Diseases. Chapter last updated: February, 2018.
FIGUEROLA-Tejerina A, Rodríguez-Caravaca G, Bustamante-Munguira et al. Epidemiological Surveillance of Surgical Site Infection and its Risk Factors in Cardiac Surgery: A Prospective Cohort Study. Rev Esp Cardiol (Engl Ed). 2016; 69(9): 842-848. doi: 10.1016/j.rec.2016.01.030Altemeier WA et al. Manual on control of infection in surgical patients. JB Lippincott 2nd Ed, Philadelphia, 1984, p 29.
GILLESPIE, Brigid M et al. “Quality appraisal of clinical guidelines for surgical site infection prevention: A systematic review.” PloS one vol. 13,9 e0203354. 13 Sep. 2018 doi: 10.1371/journal. pone.0203354Global Guidelines for the Prevention of Surgical Site Infection. (2016). Geneva: World Health Organization. Disponível em :< ttps://www.ncbi.nlm.nih.gov/books/NBK401132/>. Acesso em: 15 de dezembro de 2021.
GOMILA A et al. Risk factors and outcomes of organ-space surgical site infections after elective colon and rectal surgery. Antimicrob Resist Infect Control. 2017 Apr 21; 6:40.
HEBERT C et al. Decolonization therapy in infection control. Curr Opin Infect Dis. 2010 Aug;23(4):340-5.
Keely Boyle, K et al. “Centers for DiseaseControland Prevention 2017 Guidelines for Prevention of Surgical Site Infections: Review and Relevant Recommendations.” Current reviews in musculoskeletal medicine vol. 11,3 (2018): 357-369.
BRATZLER, D.W et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health-Syst Pharm. 2013.
LIU Z, et al. Intraoperative interventions for preventing surgical site infection: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2018.
MSSIC. Preventing Surgical Site Infections.Chicago, IL: Health Research & Educational Trust.Disponível em :<https://mssic.org/wp-content/uploads/2020/04/SSI-Change-Package-HRET-2018-Update.pdf>. Acesso em: 16 de dezembro de 2021.
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE.GuidelineSurgical site infections: prevention and treatment. Nice.org.uk/guidance/ng125. Published:11 April 2019.
OLIVEIRA, Adriana Cristina de; GAMA, Camila Sarmento. Evaluation of adherence to measures for the prevention of surgical site infections bythe surgical team. Rev. Esc. Enferm. USP, São Paulo , v. 49, n. 5, p. 767-774, Oct. 2015 .
Cruse PJ, Foord R. The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds. Surg Clin North Am. 1980 Feb. 60(1):27-40.
PENG H-M. et al. Effectiveness of preoperative decolonization with nasal povidone iodine in Chinese patients undergoing elective orthopedic surgery: a prospective cross-sectional study. Braz J Med Biol Res [Internet]. 2018 [cited 2019 May 22]; 51(2): e6736.
P. S. et al, Supplemental oxygen and surgical site infection: getting tothe truth, BJA: British Journal of Anaesthesia, Volume 119, Issue 1, July 2017, Pages 13–15,
PSNET.Health Care-Associated Infections. A Meta-analysis of Costs and Financial Impact on the US Health Care System. JAMA Intern Med, 173(22): (2013): 2039-46.
SANTOS, Maria de Lourdes Gonçalves; TEIXEIRA, Renata Rezende; DIOGO-FILHO, Augusto. Surgical site infections in adults patients undergoing of clean and contaminated surgeries at a university Brazilian hospital. Arq. Gastroenterol., São Paulo , v. 47, n. 4, p. 383-387, Dec. 2010.
SEPTIMUS EJ et al. Decolonization in Prevention of Health Care-Associated Infections. Clinical Microbiology Reviews Jan 2016, 29 (2) 201-222.
SPORER, S.M et al. L. Methicillin-Resistant and Methicillin-sensitive Staphylococcus aureus Screening and Decolonization to Reduce Surgical Site Infection in Elective Total Joint Arthroplasty. J. Arthroplasty 2016, 31, 144-147.
STEVENS M. The Operation Room – Chapter Editor. Guide to Infection Control in the Hospital. International Society for Infectious Diseases. Chapter last updated: February,2018.
SULEIMAN LI, et al. Intraoperative Considerations for Treatment/Prevention of Prosthetic Joint Infection. Curr Rev Musculoskeletal Med. 2018 Sep;11(3):401-408. Review.
SURGICAL Site Infection(SSI)Event.https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
Acesso. January 2022.
TORRES SI, et al.(2017). CDC- Center Disease of Control and Prevention. Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg, 152(8), 784-791.
Turner MC et al. Surgical Site Infection: The Clinical and Economic Impact. Clin Colon Rectal Surg. 2019 May;32(3):157-165.
Z LIU et al. Nasal decontamination for the prevention of surgical site infection in carriers. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No.: CD012462
SHEA, APIC, CDC, SIS. Consensus paper on the Surveillance of surgical Wound infections. Infect Control Hosp Epidemiol 1992; 13:599.
Cruse PJ. Surgical wound infection. In: InfectiousDiseases, Wonsiewicz MJ (Ed), WB Saunders Co, Philadelphia 1992. p.758.
Aranha JR, Aroni P, Pinhatti EDG, Ribeiro RP. Exposição à fumaça cirúrgica: como se proteger? Rev Enferm UFPE online. 2020;14:e243963.
Norma da ABNT NBR nº 7256/82 – Centro Cirúrgico – sistema de ventilação.
Azi LMTA, Fonseca NM, Linard LG. SBA 2020: Atualização das recomendações para segurança em anestesia regional [SBA 2020: Regional anesthesia Safety recommendations update]. Braz J Anesthesiol. 2020 Jul-Aug;70(4):398-418. Portuguese.
Boyce JM. Best products for skinantisepsis. Am J Infect Control. 2019 Jun;47S:A17-A22.
Wade RG, Burr NE, McCauley G, Bourke G, Efthimiou O. The ComparativeEfficacy of ChlorhexidineGluconate and Povidone-iodineAntiseptics for the Prevention of Infection in Clean Surgery: A Systematic Review and Network Meta-analysis. Ann Surg. 2021 Dec 1;274(6):e481-e488.
Ramirez Galleymore P, Viera V. Preoperativeskinantisepsis. Med Intensiva (Engl Ed). 2019 Mar;43 Suppl 1:18-22.
Shadid MB, Speth MJGM, Voorn GP, Wolterbeek N. Chlorhexidine 0.5%/70% Alcohol and Iodine 1%/70% Alcohol Both ReduceBacterialLoad in Clean Foot Surgery: A Randomized, ControlledTrial. J FootAnkle Surg. 2019 Mar;58(2):278-281.
Charles D, Heal CF, Delpachitra M, Wohlfahrt M, Kimber D, Sullivan J, Browning S, Saednia S, Hardy A, Banks J, Buttner P. Alcoholic versus aqueouschlorhexidine for skinantisepsis: the AVALANCHE trial. CMAJ. 2017 Aug 8;189(31):E1008-E1016.
Davies BM, Patel HC. Systematic Review and Meta-Analysis of Preoperative Antisepsis with Combination Chlorhexidine and Povidone-Iodine. Surg J (N Y). 2016;2(3):e70-e77. Published 2016 Aug 10.
Mario Mastrocola, Georg Matziolis, Sabrina Böhle, Chris Lindemann, Peter Schlattmann&Henk Eijer1Meta‑analysis of theefficacy of Preoperative skin preparationwith Alcoholic chlorhexidine compared to Povidone iodine in orthopaedic surgery. ScientificReports (2021) 11:18634.
Surgical site infection: prevention and treatment [B] Evidence review for theeffectiveness of skinantiseptics in the prevention of surgical site infection NICE guideline NG125 Evidence reviews April 2019.
Charehbili A, Koek MBG, de Mol van Otterloo JCA, Bronkhorst MWGA, van der Zwaal P, Thomassen B, Waasdorp EJ, Govaert JA, Bosman A, van denBremer J, Ploeg AJ, Putter H, Meijs AP, van de Velde CJH, van Gijn W, Swijnenburg RJ. Cluster-randomized crossover trial of chlorhexidine-alcohol versus iodine-alcohol for prevention of surgical-site infection (SKINFECT trial). BJS Open. 2019 May 20;3(5):617-622.
Dörfel D, Maiwald M, Daeschlein G, Müller G, Hudek R, Assadian O, Kampf G, Kohlmann T, Harnoss JC, Kramer A. Comparison of the Antimicrobial efficacy of povidone-iodine-alcohol versus chlorhexidine-alcohol for surgical skinpreparation on theaerobic and anaerobicskin flora of theshoulder region. Antimicrob Resist Infect Control. 2021 Jan 22;10(1):17.
Matthias Maiwald, Andreas F Widmer. WHO’srecommendation for surgical skinantisepsisispremature. www.thelancet.com/infection Vol 17 October 2017, 1023.
Patrick S, McDowell A, Lee A, Frau A, Martin U, Gardner E, McLorinan G, Eames N. Antisepsis of theskinbeforespinalsurgerywithpovidoneiodine-alcoholfollowedbychlorhexidinegluconate-alcohol versus povidoneiodine-alcohol Applied twice for the prevention of contamination of thewoundbybacteria: a randomized Controlled trial. Bone Joint J. 2017 Oct;99-B(10):1354-1365.
Estabelece a Lista de Medicamentos de Baixo Risco sujeitos à notificação. Diário Oficial da União. Publicado em: 12/11/2021 | Edição: 213 | Seção: 1 | Página: 129 .Órgão: Ministério da Saúde/Agência Nacional de Vigilância Sanitária/Diretoria Colegiada. Instrução normativa – in n° 106, de 11 de novembro de 2021.
de Vries EN, Prins HA, Crolla RM, den Outer AJ, van Andel G, van Helden SH, Schlack WS, van Putten MA, Gouma DJ, Dijkgraaf MG, Smorenburg SM, Boermeester MA; SURPASS Collaborative Group. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med. 2010 Nov 11;363(20):1928-37.
X NuvialisCasalsAntisepsiacutánea en losprocedimientos invasivos. Medicina Intensiva, Volume 43, Supplement 1, March 2019, Pages 35-38.
Kimberly S Johnson,Daniel J Sexton, Lumbar puncture: Technique, indications, contraindications, and complications in adults.Literature review current through: Feb 2022. |UPDODATE.
Ghobrial GM, Wang MY, Green BA, Levene HB, Manzano G, Vanni S, Starke RM, Jimsheleishvili G, Crandall KM, Dididze M, Levi AD. Preoperative skin antisepsis with chlorhexidine gluconate versus povidone-iodine: a prospective analysis of 6959 consecutive spinal surgery patients. J Neurosurg Spine. 2018 Feb;28(2):209-214.
Russo PL, Saguil E, Chakravarthy M, Lee KY, Ling ML, Morikane K, Spencer M, Danker W, Yu NYC, Edmiston CE Jr. Improving surgical site infection prevention in Asia-Pacific through appropriate surveillance programs: Challenges and recommendation. Infect Dis Health. 2021 Aug;26(3):198-207.
Worboys Michael.2013. Joseph Lister and the performance of antiseptic surgery Notes Rec. R. Soc.67199–209.
WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva: World Health Organization; 2009. 13, Surgical hand preparation: state-of-the-art.
Vermeil T et al. Hand hygiene in hospitals: anatomy of a revolution. J Hosp Infect. 2019 Apr;101(4):383-392.
Boyce JM et al. Healthcare infection control practices advisory committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task force. Guideline for hand hygiene in health-care settings. MMWR 2002; 51:1-45.
World Health Organization 2016. Global Guidelines for the Prevention of Surgical Site Infection.
Tanner J et al. Surgical hand antisepsis to reduce surgical site infection. Cochrane Database Syst Rev. 2016 Jan 22;(1):CD004288.
Jolivet S et al. Surgical field and skin preparation. OrthopTraumatol Surg Res. 2019 Feb;105(1S):S1-S6.
Centers for Disease Control and Prevention. (2018, January). Surgical Site Infection (SSI) https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf.
Fortaleza CMCB et al. Multi-state survey of healthcare-associated infections in acute care hospitals in Brazil. J Hosp Infect. 2017 Jun; 96(2): 139-1 44.
Tendência de internações e mortalidade por causas cirúrgicas no Brasil, 2008 a 2016. Rev. Col. Bras. Cir. vol.46 no.1 Rio de Janeiro 2019 Epub Feb 18, 201.
Matthew J. Javitt, BS; Association Between Eliminating Water From Surgical Hand Antisepsis at a Large Ophthalmic Surgical Hospital and Cost; JAMA Ophthalmol. 2020;138(4):382-386.
Peixoto, Juliana Gil Prates; Branco, Aline; Dias, Cícero Armídio Gomes; Millão, Luzia Fernandes; Caregnato, Rita Catalina Aquino. ANTISSEPSIA CIRÚRGICA DAS MÃOS COMPREPARAÇÃO ALCOÓLICA: REDUÇÃO MICROBIANA EMDIFERENTES TEMPOS DE USO NO CENTRO CIRÚRGICO. Rev. SOBECC ; 25(2): 83-89, 30/06/2020.
NOTA TÉCNICA Nº01/2018 GVIMS/GGTES/ANVISA: ORIENTAÇÕES GERAIS PARA HIGIENE DAS MÃOS EM SERVIÇOS DE SAÚDE.
Oliveira, A C et al.Avaliação da adesão às medidas para a prevenção de infecções do sítio cirúrgico pela equipe cirúrgica. RevEsc Enferm USP · 2015; 49(5):767-774.
Florman S et al Efficacy of double gloving with an intrinsic indicator system. Surg Infect (Larchmt). 2005;6(4):385-395.
Thomas-Copeland, Do Surgical Personnel Really Need to Double-Glove? AORN Journal, FEBRUARY 2009, VOL 89, NO 2; page 327.
Centers for Disease Control and Prevention. Guideline for prevention of surgical site infection,19 99. Infection Control and Hospital Epidemiology, April 1999, 20(4):247 278.
OSHA Technical Manual Section VI: Chapter 1. Hospital Investigations: Health Hazards.Available at https://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_1.html.
AORN Guideline for Sterile Technique from 2015 Guidelines for Perioperative Practice.
Statement on Sharps Safety. American College of Surgeons. October 2007. https://www.facs.org/about-acs/statements/58-sharps-safety.
“Information Statement 1018: Preventing the Transmission of Bloodborne Pathogens. “American Academy of Orthopaedic Surgeons. http://www.aaos.org/news/aaosnow/oct13/clinical5.asp.
Leading the way in best Practice. International College of Surgeons. Available at: https://www.icsglobalorg/members/sections/mem_sect_news_europe.asp.
Glove Use Information Leaflet. World Health Organization. August 2009. Available at: http:// www.who.int/gpsc/5may/Glove_Use_Information_Leaflet.pdf.
Childs T. Use of double gloving to reduce surgical personnel’s risk of exposure to bloodborne pathogens: an integrative review. AORN. 2013;98(6):585-596.
Cochrane Database of Systematic Reviews. 2006, Issue 3 Art. No.: CD003087. DOI: 10.1002/146518 58.CD003087.pub.
Korniewicz D et al.Exploring the benefits of double gloving during surgery. AORN J. 2012;95:328-336.4
Kaya I et al. Glove perforation time and frequency in total hip arthroplasty procedures. Acta OrthopTraumatolTurc. 2012;46(1):57-60.
Timler D et al. Glove failure in elective thyroid surgery. A prospective randomized study. International Journal of Occupational Medicine and Environmental Health. 2015;28(3).
Kumar D et al. Cross-sectional Analysis ofGlove Perforation in Primary and Revision Total Hip Arthroplasty. Malays OrthopJ. 2016 Nov;10(3):31-35.
Kristen K A. al. Executive Summary of the American College of Surgeons/Surgical Infection Society Surgical Site Infection Guidelines—2016 Update.SURGICAL INFECTIONS Volume 18, Number 4, 2017.
Centers for Disease Control and Prevention (CDC). (2008). Workbook for Designing, Implementing and Evaluating a SharpsInjury Prevention Program.
http://www.cdc.gov/sharpssafety/pdf/sharpsworkbook_2008.pdf.
Zhang Z, Gao X, Ruan X, Zheng B. Effectiveness of double-glovingmethod on prevention of surgical gloveperforations and blood contamination: A systematic review and meta-analysis. J AdvNurs. 2021 Sep;77(9):3630-3643.
Mischke C, Verbeek JH, Saarto A, Lavoie MC, Pahwa M, Ijaz S. Gloves, extra glovesorspecialtypes of gloves for preventingpercutaneousexposure injuries in healthcare personnel. Cochrane Database Syst Rev. 2014 Mar 7;(3):CD009573.
BARBOSA, L. M.; PEIXOTO, S. S. .; MONTEIRO, J. L. G. C.; CARNEIRO, C. D. A.; OLIVEIRA, L. M. L. de; RAMOS, A. C.; ARAÚJO, G. M.; BESSA NOGUEIRA, R. V. .; LAUREANO FILHO, J. R.; VASCONCELOS, B. C. do E. Surgical gloveperforationduring oral and maxillofacial surgical procedures: An experimental study. Research, Society and Development, [S. l.], v. 10, n. 6, p. e2610615290, 2021.
Enz A, Kamaleddine I, Groß J, Schafmayer C, Alwafai E, Sievers L, Mittelmeier W, Klinder A. Is Single Gloving Still Acceptable? Investigation and Evaluation of Damages on SterileLatexGloves in General Surgery. J Clin Med. 2021 Aug 29;10(17):3887.
